Thyroid Hemiagenesis: A Single-Center Case Series
Abstract
Introduction: Thyroid hemiagenesis (TH) is a rare congenital anomaly characterized by the complete absence of one thyroid lobe, with or without absence of the isthmus. Its etiology remains unclear, and epidemiological data are limited. Although TH is often asymptomatic and discovered incidentally, it may pose clinical challenges when accompanied by thyroid dysfunction or structural abnormalities. This study reviews a single-center experience in diagnosing this condition and highlights its clinical significance..
Methods: This single-center case series was conducted from July 2021–July 2024, analyzing TH cases confirmed via ultrasonography. Eligible patients had complete medical records, including demographics, clinical presentation, radiological findings, and thyroid function status. Data were retrieved from electronic records and analyzed using SPSS 27.0, employing descriptive statistics to summarize means, ranges, frequencies, and percentages, ensuring a comprehensive assessment of TH’s clinical and epidemiological characteristics..
Results: This study analyzed 11 patients with TH (mean age: 28.12 ± 18.14 years; range: <1–55 years), seven of whom were females (63.6%). The diagnosis was incidental in six cases (54.5%), while three (27.3%) presented with neck swelling and two (18.2%) with neck pain. Thyroid function was euthyroid in seven (63.6%), hyperthyroid in two (18.2%), and hypothyroid in two (18.2%). Ultrasound examination confirmed left lobe and isthmus agenesis in eight cases (72.7%). Follow-up ranged from 4 to 48 months.
Conclusion: This study confirms the female predominance of TH, with left-lobe absence being the most common. Congenital anomalies suggest embryological links. While thyroid function is typically preserved, those with hypo- and hyperthyroidism highlight the need for individualized endocrine assessment and monitoring.
Introduction
Thyroid hemiagenesis (TH), first described in 1852, is characterized by the absence of one thyroid lobe, with or without the isthmus. It is typically detected incidentally during neck imaging, as most affected individuals remain asymptomatic and undiagnosed. However, epidemiological studies suggest a higher occurrence in regions endemic for hypothyroidism, potentially indicating an underlying environmental or genetic predisposition [1].
Epidemiological analyses reveal distinct anatomical patterns in TH. Approximately 80% of documented cases involve agenesis of the left thyroid lobe, establishing a left-to-right prevalence ratio of 4:1. When the left lobe is absent, the isthmus is missing in nearly half of cases [2]. In contrast, right lobe agenesis is more frequently associated with complete isthmus absence. Additionally, a well-documented female predominance exists, though the mechanisms contributing to this gender disparity remain unclear [3].
The pathogenesis of TH likely results from disruptions in key developmental processes, including defective migration, differentiation, or proliferation of thyroid precursor cells. Normal thyroid development begins in the fourth gestational week as an endodermal outpouching from the pharyngeal floor, which elongates into a bilobed structure and descends to its final position in the neck [3]. Any disturbance in this sequence can lead to congenital thyroid anomalies, with TH being one of the rarer manifestations. TH remains underreported due to its typically asymptomatic nature compared to more commonly recognized thyroid malformations, such as thyroglossal duct cysts or ectopic thyroid tissue. This underscores the need for systematic studies to assess its true prevalence and clinical significance [4].
The precise molecular mechanisms underlying TH remain to be understood completely, though evidence suggests a multifactorial etiology involving genetic and environmental influences. Genetic analyses of thyroid dysgenesis have identified key regulatory genes, FOXE1, PAX8, NKX2-1, NKX2-5, and TSHR, which play crucial roles in thyroid organogenesis [3]. While TH is primarily considered a sporadic anomaly, familial clustering, in some cases, suggests the possibility of heritable genetic influences. However, establishing definitive genotype-phenotype correlations remains challenging, as many cases occur in isolation without clear inheritance patterns. The interplay between genetic susceptibility and developmental signaling pathways continues to be an area of ongoing research [5].
Although numerous case reports exist, large-scale case series on TH remain scarce. The rarity of the condition, coupled with its typically benign and asymptomatic presentation, has contributed to a gap in comprehensive epidemiological and developmental studies. This study aims to review a single-center experience in diagnosing and managing TH cases. Additionally, all referenced sources have undergone verification [6].
Methods
Study design and Setting
This study was conducted as a single-center case series at the Thyroid Clinic of Smart Health Tower. The study period extended from July 2021 to July 2024, during which all eligible patients diagnosed with TH were identified and analyzed. The clinic serves as a specialized referral center for thyroid disorders, ensuring comprehensive diagnostic evaluation and follow-up of affected individuals.
Participant Selection and Eligibility Criteria
The study included all patients with a confirmed diagnosis of TH based on ultrasonographic imaging. Patients were eligible for inclusion if they had complete medical records detailing their demographic characteristics, clinical presentation, and radiological findings. Cases with incomplete data, particularly those lacking essential imaging reports or follow-up details, were excluded to ensure consistency and reliability in the analysis.
Data Collection and Variables Assessed
Patient data were systematically retrieved from the hospital’s electronic medical records, radiology reports, and clinicaldocumentation. The collected variables included demographic characteristics such as age, sex, residency, and clinical presentation, including symptoms at diagnosis and the presence of thyroid dysfunction or associated comorbidities. Medical and surgical history, including prior thyroid conditions and interventions, was also documented. Radiological findings focused on the laterality of TH, the size of the contralateral lobe, and any evidence of compensatory hypertrophy. Additionally, laboratory investigations, including thyroid function tests (thyroid stimulating hormone (TSH), triiodothyronine (T3), and thyroxine (T4) levels), were analyzed to assess thyroid function status. Follow-up data were reviewed to evaluate disease progression, changes in thyroid function, and any medical or surgical interventions undertaken.
Data Processing and Statistical Analysis
All collected data were documented and organized using Microsoft Excel 2021. Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 27.0. Descriptive statistical methods were employed to summarize the findings, with continuous variables presented as mean and range, while categorical variables were expressed as frequencies and percentages.
Results
This study included 11 patients diagnosed with TH, with a mean age of 28.12 ± 18.14 years (range: <1 to 55 years). The cohort comprised seven females (63.6%) and four males (36.4%). Clinically, six patients (54.5%) were diagnosed incidentally, three (27.3%) presented with neck swelling, and two (18.2%) reported neck pain. Regarding associated congenital conditions, eight patients (72.7%) had no additional anomalies. Among the remaining three, one had a thyroglossal duct cyst, one had a history of prolonged neonatal jaundice, and another presented with both prolonged neonatal jaundice and a periumbilical hernia.
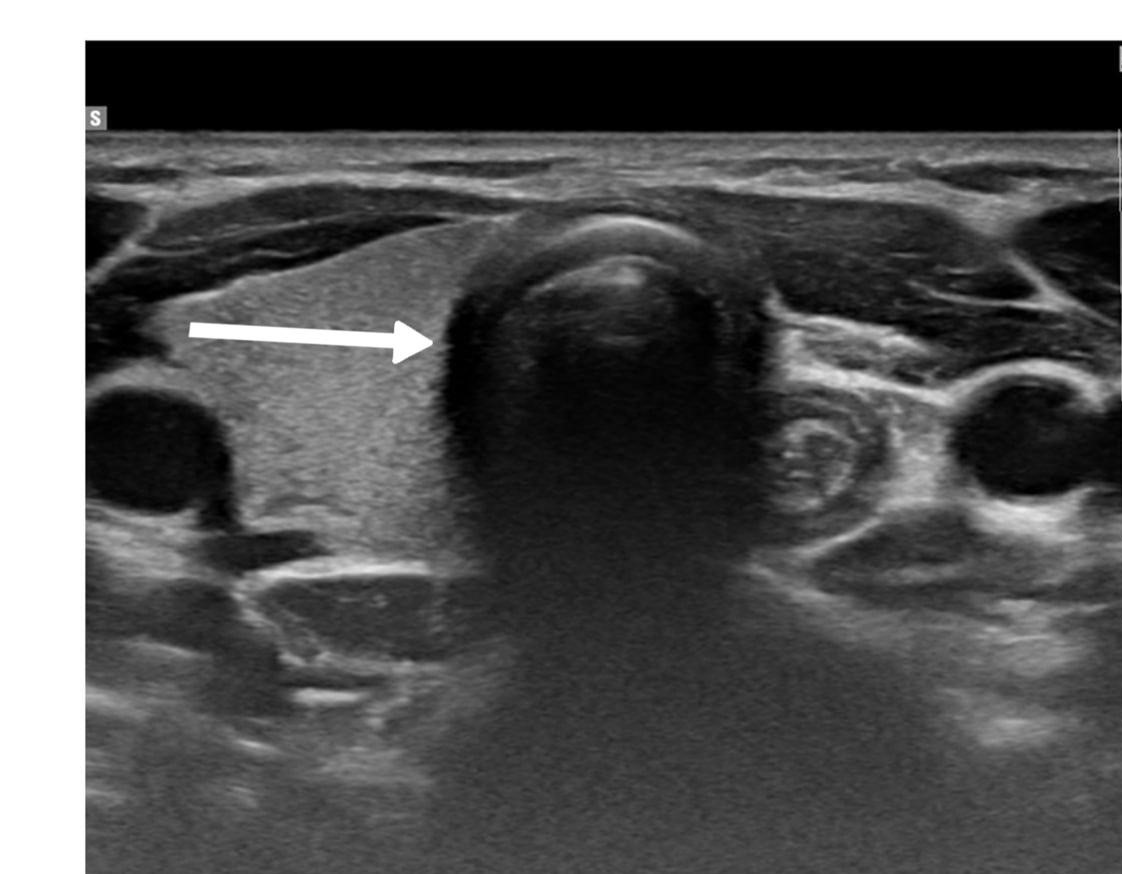
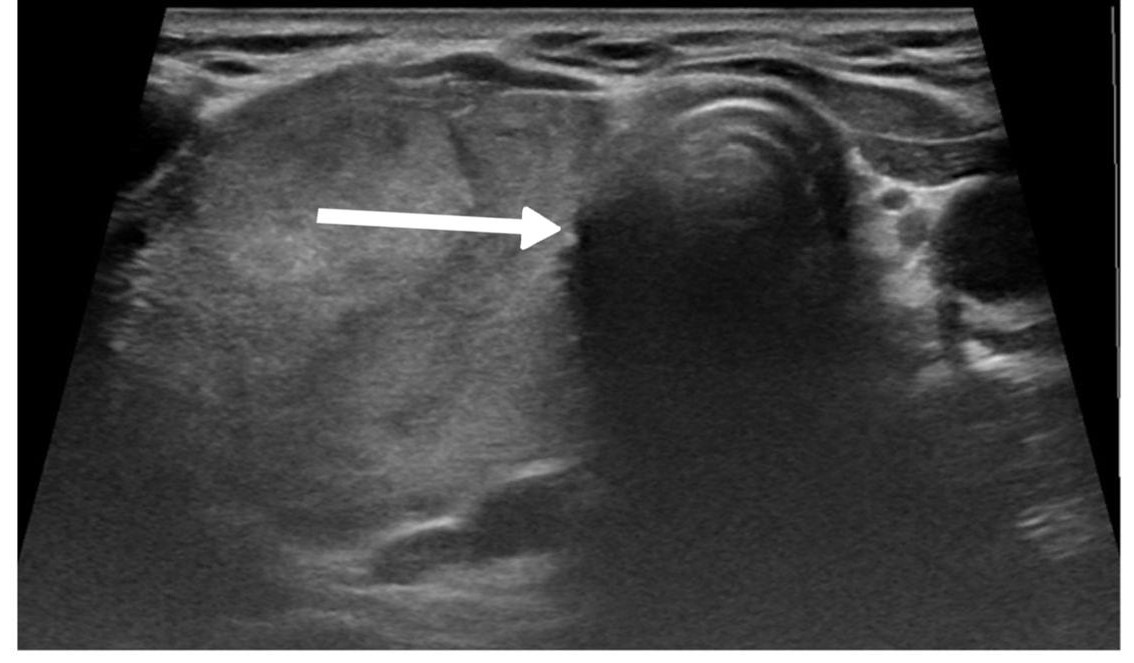
Thyroid function assessment revealed that the majority (7 cases ,63.6%) of patients were euthyroid (0.35-4.5µIU/mL), while two patients exhibited hyperthyroidism (<0.35 µIU/mL), and two of them had hypothyroidism (>4.5 µIU/mL). Ultrasound findings demonstrated left lobe and isthmus agenesis in eight cases (72.7%), while two cases (18.2%) exhibited isolated left lobe agenesis, and one patient exhibited isolated right lobe agenesis (9.1%) (Figures 1 and 2). The largest documented normal lobe measured 100 × 43 × 35 mm, whereas the smallest measured 15 × 6.9 × 7.9 mm. The follow-up period ranged from 4 to 48 months (Tables 1-3).
|
Cases |
Age (Y) |
Gender |
History |
Clinical Thyroid Examinations
|
|||||
|
Presentation |
Duration (W) |
Other Congenital Conditions |
PMH |
PSH |
Drug Hx |
||||
|
Case 1 |
20 |
M |
Neck Swelling |
4 |
None |
Hyperthyroidism |
Negative |
Methimazole |
G2 |
|
Case 2 |
21 |
F |
Neck Pain |
3 |
None |
Negative |
Lymph Node Biopsy |
None |
G2 |
|
Case 3 |
16 |
F |
Incidental |
8 |
Jaundice, Periumbilical Hernia |
Iron Deficiency Anaemia |
Hernia Surgery |
None |
G0 |
|
Case 4 |
<1 |
M |
Incidental |
N/A |
Jaundice |
Hypothyroidism |
Negative |
Thyroxine |
G1 |
|
Case 5 |
31 |
M |
Incidental |
N/A |
None |
Negative |
Negative |
None |
G0 |
|
Case 6 |
55 |
F |
Neck Pain |
8 |
None |
Negative |
Bilateral Total Knee Replacement |
None |
G0 |
|
Case 7 |
26 |
F |
Neck Swelling |
1 |
None |
Negative |
Tonsillectomy |
None |
G2 |
|
Case 8 |
6 |
F |
Neck Swelling |
1 |
Thyroglossal Duct Cyst |
Negative |
Tonsillectomy |
None |
G2 |
|
Case 9 |
55 |
F |
Incidental |
N/A |
None |
Negative |
C-section |
None |
G0 |
|
Case 10 |
53 |
F |
Incidental |
1 |
None |
Negative |
C-section |
None |
G0 |
|
Case 11 |
27 |
M |
Incidental |
3 |
None |
Negative |
Negative |
None |
G0 |
|
M: Male, F: Female, PMH: Past Medical History, PSH: Past Surgical History, Drug Hx: Drug History, G: grades of thyroid enlargement, Y: Years, W: Weeks. |
|||||||||
|
Cases |
Blood Investigations |
Ultrasound Reports |
Follow up (Months) |
|||||
|
First TSH (µIU/mL) |
First Free T4 (pmol/L) |
First Total T4 (nmol/mL) |
ATPO (IU/mL) |
First TRAB (IU/mL) |
Agenesis Side |
Normal Lobe Size (mm) |
||
|
Case 1 |
0.005 |
55.53 |
N/A |
N/A |
12.55 |
Left Lobe, Isthmus |
70 × 26 × 24 |
48 |
|
Case 2 |
2.45 |
19.9 |
N/A |
9 |
N/A |
Left Lobe, Isthmus |
56 × 19 × 19 |
12 |
|
Case 3 |
1.8 |
N/A |
165.6 |
N/A |
N/A |
Left Lobe, Isthmus |
53 × 16 × 14 |
12 |
|
Case 4 |
100 |
2.34 |
N/A |
N/A |
N/A |
Left Lobe |
15 × 6.9 × 7.9 |
8 |
|
Case 5 |
2.61 |
18.00 |
N/A |
68.3 |
N/A |
Left Lobe, Isthmus |
100 × 43 × 35 |
12 |
|
Case 6 |
1.83 |
15.86 |
N/A |
109.2 |
N/A |
Left Lobe, Isthmus |
64 × 31 × 27 |
4 |
|
Case 7 |
1.27 |
16.3 |
N/A |
80.9 |
N/A |
Left Lobe, Isthmus |
55 × 19 × 19 |
6 |
|
Case 8 |
1.33 |
21.71 |
N/A |
N/A |
N/A |
Left Lobe, Isthmus |
34 × 10 × 13 |
36 |
|
Case 9 |
2.27 |
17.02 |
N/A |
11.45 |
N/A |
Left Lobe |
48 × 16 × 15 |
48 |
|
Case 10 |
5.66 |
9.75 |
N/A |
17.6 |
N/A |
Left Lobe, Isthmus |
48 × 18 × 20 |
4 |
|
Case 11 |
0.005 |
41.6 |
9.75 |
N/A |
0.8 |
Right Lobe |
58 × 26 × 25 |
24 |
|
TSH: Thyroid-Stimulating Hormone, T4: Thyroxine, ATPO: Anti-Thyroid Peroxidase Antibodies, TRAB: Thyrotropin Receptor Antibodies, N/A: Not Applicable |
||||||||
|
Variables |
Frequency (percentage) |
|
Gender Male Female |
4 (36.4%) 7 (63.6%) |
|
Age, Years (Mean ± SD) |
28.12 ± 18.14 |
|
Clinical presentations Incidental Neck Pain Neck Swelling |
6 (54.5%) 2 (18.2%) 3 (27.3%) |
|
Past medical history Iron Deficiency Anemia Negative |
1 (9.1%) 10 (90.9%) |
|
Clinical thyroid examination G0 G1 G2 |
6 (54.5%) 1 (9.1%) 4 (36.4%) |
|
Thyroid function status Euthyroid Hyperthyroidism Hypothyroidism |
7 (63.6%) 2 (18.2%) 2 (18.2%) |
|
Agenesis Side Right Left |
1 (9.1%) 10 (90.9%) |
Discussion
The clinical presentation of TH is predominantly asymptomatic, with most cases identified incidentally during imaging studies performed for unrelated thyroid conditions or neck abnormalities. When symptomatic, manifestations typically arise from concurrent thyroid disorders rather than the hemiagenesis itself. These may include neck swelling due to compensatory hypertrophy of the remaining lobe, thyroid dysfunction, or palpable nodules. The prevalence of thyroid abnormalities in individuals with TH appears to increase with age, likely due to chronic overstimulation of the remaining lobe by TSH, a factor contributing to the ongoing debate regarding the benign nature of the condition [7].
In a study in which 40 patients newly diagnosed with TH, aged between 12 and 79, were enrolled, it was found that 90% of their cohort were clinically asymptomatic regarding hemiagenesis itself, with associated conditions including euthyroid nodular goiters, multinodular goiters, Graves’ disease, and Hashimoto’s thyroiditis [8]. Another study emphasized that even symptomatic cases typically arise from coexisting thyroid pathologies rather than the anatomical defect itself [3]. Rare presentations such as hypothyroidism with prolonged neonatal jaundice and umbilical hernia have been documented in pediatric cases [3]. In the present study, 54.5% of patients were diagnosed incidentally, a lower rate than previously reported in the literature. A notable proportion exhibited clinical symptoms, with neck swelling in 27.3% and neck pain in 18.2% of cases, suggesting potential variations in clinical presentation, particularly among younger populations. Furthermore, congenital anomalies, including thyroglossal duct cyst, prolonged neonatal jaundice, and periumbilical hernia, were observed in 27.3% of cases, findings not prominently reported in earlier studies. These variations highlight the need for further investigation into potential demographic and pathophysiological factors influencing the clinical spectrum of TH.
The diagnosis of TH is mainly based on imaging modalities, with ultrasonography as the first-line investigation and thyroid scintigraphy as a complementary confirmatory tool. Ultrasound imaging is particularly valuable as the initial screening method due to its wide availability, lack of radiation exposure, and sensitivity in detecting the absence of a thyroid lobe and any structural changes in the remaining thyroid tissue [3]. Thyroid scintigraphy using technetium or iodine provides functional anatomical assessment with the advantage of detecting ectopic thyroid tissue and diagnosing concurrent thyroid pathologies in the remaining lobe. Combining these two imaging modalities remains essential for accurate diagnosis and differentiation from other conditions that might mimic hemiagenesis [3]. A large cohort case-control study by Ruchala et al. emphasizes the need for both ultrasonography and scintigraphy to distinguish true hemiagenesis from pseudoagenesis, which can occur in cases of severe atrophy or destruction of thyroid tissue [8]. In a study focused on pediatric cases with suspected thyroid dysgenesis, researchers utilized both thyroid scanning and ultrasonography to establish definitive diagnoses, with hemiagenesis identified in one of their subjects [9]. Another case report of a rare male pediatric patient with TH demonstrated how ultrasonography revealed the absence of the left lobe while the right lobe showed minimal hyperplasia without nodules; scintigraphy confirmed these findings and ruled out ectopic thyroid tissue. This case emphasized that when only one thyroid lobe is detected initially, physicians should consider TH and employ both imaging modalities before invasive procedures [10].
A retrospective evaluation of imaging for congenital hypothyroidism revealed that compared to 99mTc-pertechnetate scanning, ultrasound examination demonstrated 100% specificity but only 44% sensitivity for detecting thyroid abnormalities. This finding highlights the value of scintigraphy as a complementary method to ultrasound examination, particularly when ectopic thyroid tissue is suspected. The limitations of relying solely on ultrasonography were further illustrated in cases where thyroid agenesis was diagnosed with ultrasonography, but follow-up scintigraphy revealed sublingual thyroid tissue in a significant proportion of patients [11]. In the current study, ultrasonography was the primary diagnostic tool, revealing left lobe and isthmus agenesis in eight (72.7%) cases, while two cases (18.2%) exhibited isolated left lobe agenesis with preservation of the isthmus, and one case showed isolated right lobe agenesis with preserved isthmus (9.1%). The ultrasound findings documented normal lobe dimensions ranging from the smallest at 15 × 6.9 × 7.9 mm to the largest at 100 × 43 × 35 mm, providing valuable reference values for assessing potential compensatory hypertrophy.
Typically, TH is associated with normal thyroid function, as the remaining lobe compensates for the absent tissue. Most patients remain euthyroid, though biochemical patterns may reveal elevated TSH levels despite normal peripheral hormone concentrations, suggesting mild subclinical hypothyroidism or compensatory stimulation of the intact lobe. Functional thyroid disorders such as hyperthyroidism or hypothyroidism may coexist, often linked to concurrent pathologies like autoimmune thyroiditis or nodular goiter [3,12]. Recent studies highlight these trends. Ruchała et al. noted that while TSH and free T3 levels were elevated in TH patients compared to controls, most maintained euthyroidism [8]. Maiorana et al. documented subclinical hypothyroidism in pediatric cases [13]. Genetic factors, including potential PAX8 or FOXE1 gene involvement, may influence thyroid development but do not directly correlate with hormonal status [14]. Management focuses on addressing associated thyroid disorders rather than the anatomical defect itself. For asymptomatic patients, periodic monitoring with ultrasonography and thyroid function tests suffices [3,12]. Surgical intervention is indicated for malignancies or symptomatic nodules in the remaining lobe, necessitating lifelong thyroxine supplementation post-resection [14]. The current study aligns with these findings since 63.6% of patients were euthyroid, and only two cases of hyperthyroidism and two of hypothyroidism were identified, each requiring specific targeted therapy. While compensatory hypertrophy was observed, no evidence of progressive dysfunction emerged during follow-up, reinforcing the conservative approach for uncomplicated TH [3].
The follow-up and outcome of TH primarily focus on monitoring for potential thyroid pathologies and ensuring optimal thyroid function. Since TH itself is generally asymptomatic, the clinical significance lies in its association with other thyroid disorders. Therefore, regular follow-ups with thyroid function tests and ultrasonography are crucial to detect emerging thyroid conditions early. Recent studies emphasize the importance of long-term monitoring. For instance, a study by Peteiro-Gonzalez et al. highlighted that patients with TH are more prone to autoimmune thyroid disease and nodular goiter due to sustained compensatory stimulation of the remaining lobe, necessitating regular surveillance to manage these conditions effectively [12]. Another study suggested that patients with TH might benefit from thyroxine therapy to normalize TSH levels and potentially prevent associated thyroid pathologies. However, further research is needed to confirm this approach. In cases where TH coexists with malignancies like medullary thyroid cancer, follow-up involves regular biochemical monitoring (calcitonin and carcinoembryonic antigen levels) and ultrasonography to detect recurrence early [15]. The current study's follow-up period ranged from 4 to 48 months, with no significant thyroid dysfunction or complications reported during this time. The study's findings align with previous literature in emphasizing the need for ongoing surveillance to manage potential thyroid-related issues in patients with TH. Despite the absence of severe complications during the study period, the importance of continued monitoring cannot be overstated, given the potential for future development of thyroid pathologies in these patients.
One of the primary limitations of this study is the unavailability of advanced diagnostic tools such as thyroid scintigraphy and molecular genetic testing. Scintigraphy, which is considered the complementary tool for confirming thyroid hemiagenesis, was not performed in any of the cases due to lack of access to nuclear medicine facilities. However, all cases were assessed using high-resolution ultrasonography performed by experienced clinicians in a high-volume, thyroid-specialized center, supporting the reliability of the diagnoses. Similarly, molecular or genetic analyses that could provide insights into potential hereditary or developmental mechanisms were not feasible, primarily due to financial constraints and limited infrastructure in the setting of a developing country.
Conclusion
This study confirms the female predominance of TH, with a higher prevalence of left-lobe absence and frequent symptomatic presentations. The association with congenital anomalies suggests embryological links requiring further exploration. While thyroid function is generally preserved, cases of hypo- and hyperthyroidism underscore the need for individualized endocrine evaluation.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Ethical approval for this study was obtained from the Ethics Committee of the Kscien Organization (Approval No. 2025-37)
Consent for participation: Not applicable.
Consent for publication: Written informed consent for publication was obtained from the patients or, in the case of minors, from their parents.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: AMS, HOB, ShFA, and AMM: Major contributors to the conception of the study, as well as the literature search for related studies, and manuscript writing. KMS, AQQ, SHH, HAA, AJQ and ROM: Literature review, design of the study, critical revision of the manuscript, and processing of the tables. ANQ, AHA, DHH, and RRR: Literature review, processing of the figures, data analysis and interpretation.
Use of AI: ChatGPT-3.5 was used to assist with language refinement and improve the overall clarity of the manuscript. All content was thoroughly reviewed and approved by the authors, who bear full responsibility for the final version.
Data availability statement: Not applicable.
References
- Szczepanek-Parulska E, Zybek-Kocik A, Wartofsky L, Ruchała M. Thyroid hemiagenesis: incidence, clinical significance, and genetic background. The Journal of Clinical Endocrinology & Metabolism. 2017;102(9):3124-37. doi:10.1210/jc.2017-00784
- Jha PS, Rote-Kaginalkar V, Titare P, Jadhav MB. Hemiagenesis of thyroid with dual thyroid ectopia: A rare case report. Indian Journal of Radiology and Imaging. 2018;28(01):14-7. doi:10.4103/ijri.IJRI_292_17
- Lesi OK, Thapar A, Appaiah NN, Iqbal MR, Kumar S, Maharaj D, et al. Thyroid hemiagenesis: narrative review and clinical implications. Cureus. 2022;14(2). doi:10.7759/cureus.22401
- Faulkner J, Varadharajan K, Choudhury N. A UK reported case of Graves’ disease with thyroid hemiagenesis. BMJ Case Reports CP. 2019;12(8): e228094. doi:10.1136/bcr-2018-228094
- Szczepanek E, Ruchala M, Szaflarski W, Budny B, Kilinska L, Jaroniec M, et al. FOXE1 polyalanine tract length polymorphism in patients with thyroid hemiagenesis and subjects with normal thyroid. Hormone research in paediatrics. 2011;75(5):329-34. doi:10.1159/000322874
- Abdullah HO, Abdalla BA, Kakamad FH, Ahmed JO, Baba HO, Hassan MN, et al. Predatory Publishing Lists: A Review on the Ongoing Battle Against Fraudulent Actions. Barw Med J. 2024;2(2):26-30. doi:10.58742/bmj.v2i2.91
- Yankov YG, Stoev L, Dimanov S, Stoeva M, Stanislavova K. A rare case of papillary thyroid carcinoma in the thyroglossal duct cyst of a 14-year-old female patient with left thyroid hemiagenesis. Cureus. 2023;15(11). doi:10.7759/cureus.49712
- Ruchala M, Szczepanek E, Szaflarski W, Moczko J, Czarnywojtek A, Pietz L, et al. Increased risk of thyroid pathology in patients with thyroid hemiagenesis: results of a large cohort case–control study. European Journal of Endocrinology. 2010;162(1):153-60. doi:10.1530/eje-09-0590
- Chun S, Lee YS, Yu J. Thyroid imaging study in children with suspected thyroid dysgenesis. Annals of Pediatric Endocrinology & Metabolism. 2021;26(1):53-9. doi:10.6065/apem.2040120.060
- Ayaz ÜY, Ayaz S, Döğen ME, Api A. Ultrasonographic and scintigraphic findings of thyroid hemiagenesis in a child: report of a rare male case. Case Reports in Radiology. 2015;2015(1):917504. doi:10.1155/2015/917504
- Supakul N, Delaney LR, Siddiqui AR, Jennings SG, Eugster EA, Karmazyn B. Ultrasound for primary imaging of congenital hypothyroidism. American Journal of Roentgenology. 2012;199(3):W360-6. doi:10.2214/ajr.11.7905
- Peteiro-Gonzalez D, Cabezas-Agricola JM, Casanueva FF. Thyroid hemiagenesis: Report of five cases and literature review. Endocrinol Nutr. 2013;60:e15–e17. doi:10.1016/j.endoen.2013.10.004
- Maiorana R, Carta A, Floriddia G, Leonardi D, Buscema M, Sava L, et al. Thyroid hemiagenesis: prevalence in normal children and effect on thyroid function. The Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1534-6. doi:10.1210/jc.2002-021574
- Campennì A, Giovinazzo S, Curtò L, Giordano E, Trovato M, Ruggeri RM, et al. Thyroid hemiagenesis, Graves’ disease and differentiated thyroid cancer: a very rare association: case report and review of literature. Hormones. 2015;14:451-8. doi:10.14310/horm.2002.1606
- Li C, Zhang C, Ma C, Wang Y, Duan Q. Synchronous thyroid medullary cancer and thyroid hemiagenesis: A case report. Experimental and Therapeutic Medicine. 2025;29(4):77. doi:10.3892/etm.2025.12827
Copyright (c) 2025 The Author(s)

This work is licensed under a Creative Commons Attribution 4.0 International License.


